
Presented here is I was able to piece together, starting with the introduction of three elements.
- Nitrogen (N)
- Oxygen (O)
- Hydrogen(H)
and three types of molecules, made up of these elements.
- Nitrogen (N₂): Two nitrogen atoms bonded together, very stable, makes up 78% of air
- Nitrates (NO₃⁻): Nitrogen combined with oxygen, forming an ion that dissolves in water
- Ammonia (NH₃): Nitrogen combined with hydrogen
Everything starts with the fact that plants need nitrogen. But dang it, they can’t get it from N₂ molecules because those molecules are too stable for plants to break down which is too bad because that’s what makes up 78% of the air.But all is not lost because plants CAN get nitrogen from nitrates and ammonia. These two molecules are made available through a natural process called Nitrogen Fixation in which bacteria converts unusable (N₂) molecules from the air, water and earth into ammonia. There is also another natural process called Organic Decomposition where nitrogen that is already in biological compounds like protein and DNA is converted into ammonia AND nitrates.
But these two processes have their limits. Nitrogen Fixation, which effectively moves nitrogen from the atmospheric reservoir to the biological reservoir, is very slow and limited. And organic decomposition is basically just recycling nitrogen that’s already in the biological reservoir is limited to what is already there. This is why agriculture has historically been nitrogen-constrained despite the abundance of nitrogen all around us. It’s matter of what kind of molecule the nitrogen is in.
Still, it’s estimated that all natural sources including nitrogen fixation, organic decomposition and nitrate deposits may only support 2-3 billion people at most. But then came perhaps the most influential invention of the 20th century… The Haber-Bosch process.

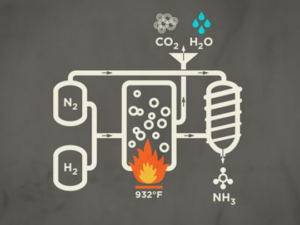
This is done by combining the N₂ molecules with H₂ molecules found in hydrocarbons like natural gas, oil or coal.By products of the process are carbon dioxide (CO₂) and water vapor (H₂O).

|
The Haber-Bosch process effectively unlocks nitrogen from stable N₂ molecules and makes it available to plants by transferring it to ammonia molecules (NH₃).
This is done by combining the N₂ molecules with H₂ molecules found in hydrocarbons like natural gas, oil or coal.By products of the process are carbon dioxide (CO₂) and water vapor (H₂O). |
This process created a whole new industry that fed the fertilizer industry with ammonia that not only replaced the need for natural nitrates like guano, it also put agriculture on steroids inadvertently inflating the world population by more than 5 billion people.
If this was still the 60’s, Disneyland still hand something to do with science and exploration and I was still an optimistic young lad, I would say this is great! No one has to starve. What a cool invention. But that was when I was still impressionable and it was before the oil peak.
Now that I am a jaded old cankerous cuss, weatherbeaten from decades of media exposure on corporate-sponsored inhumanity, I’m more likely to ask, “What are we going to do if for some reason, our supply-chain is disrupted?